Tirzepatide 101: Uses, Benefits, Side Effects, and Comparison to Semaglutide
Tirzepatide

Eileen Quinones
•
10 mins read
• Oct 7, 2024
Tirzepatide is gaining attention for its effectiveness in managing type 2 diabetes and supporting weight loss. This blog will walk you through what tirzepatide is, how it works, its benefits, side effects, and how it compares to other medications like semaglutide. Whether you're considering this medication for diabetes management or weight loss, here's everything you need to know about the peptide.
What is Tirzepatide?
Tirzepatide is a synthetic antidiabetic drug used to treat type 2 diabetes. It belongs to a new class of drugs called dual GLP-1 and GIP receptor agonists. This means it activates two types of hormone receptors:
GLP-1 (glucagon-like peptide-1)
GIP (glucose-dependent insulinotropic peptide)
These hormones, called incretins, help your body release insulin when blood sugar is high. Because it acts on two receptors, tirzepatide is often referred to as a "twincretin."
Tirzepatide was approved by the FDA in 2022 under the brand name Mounjaro. It is administered via an injection under the skin once a week.
History of Tirzepatide
Tirzepatide was developed by Eli Lilly for managing type 2 diabetes and weight loss. The drug was first patented in 2016, and after successful trials, Eli Lilly applied for FDA approval in October 2021. By April 2022, tirzepatide was shown to help not just diabetes patients but also overweight and obese individuals—regardless of whether they had diabetes.
Clinical Trials and FDA Approval
Tirzepatide received FDA approval based on data from nine clinical trials involving over 7,700 participants with type 2 diabetes. These trials were conducted worldwide, including in the U.S., Brazil, Japan, and the EU.
Five trials focused on how well tirzepatide controlled blood sugar.¹
Four trials focused on its safety.²
Participants were given tirzepatide, a placebo, or other diabetes medications. These trials lasted between 40 to 104 weeks, measuring the reduction in HbA1c, which tracks long-term blood sugar control.
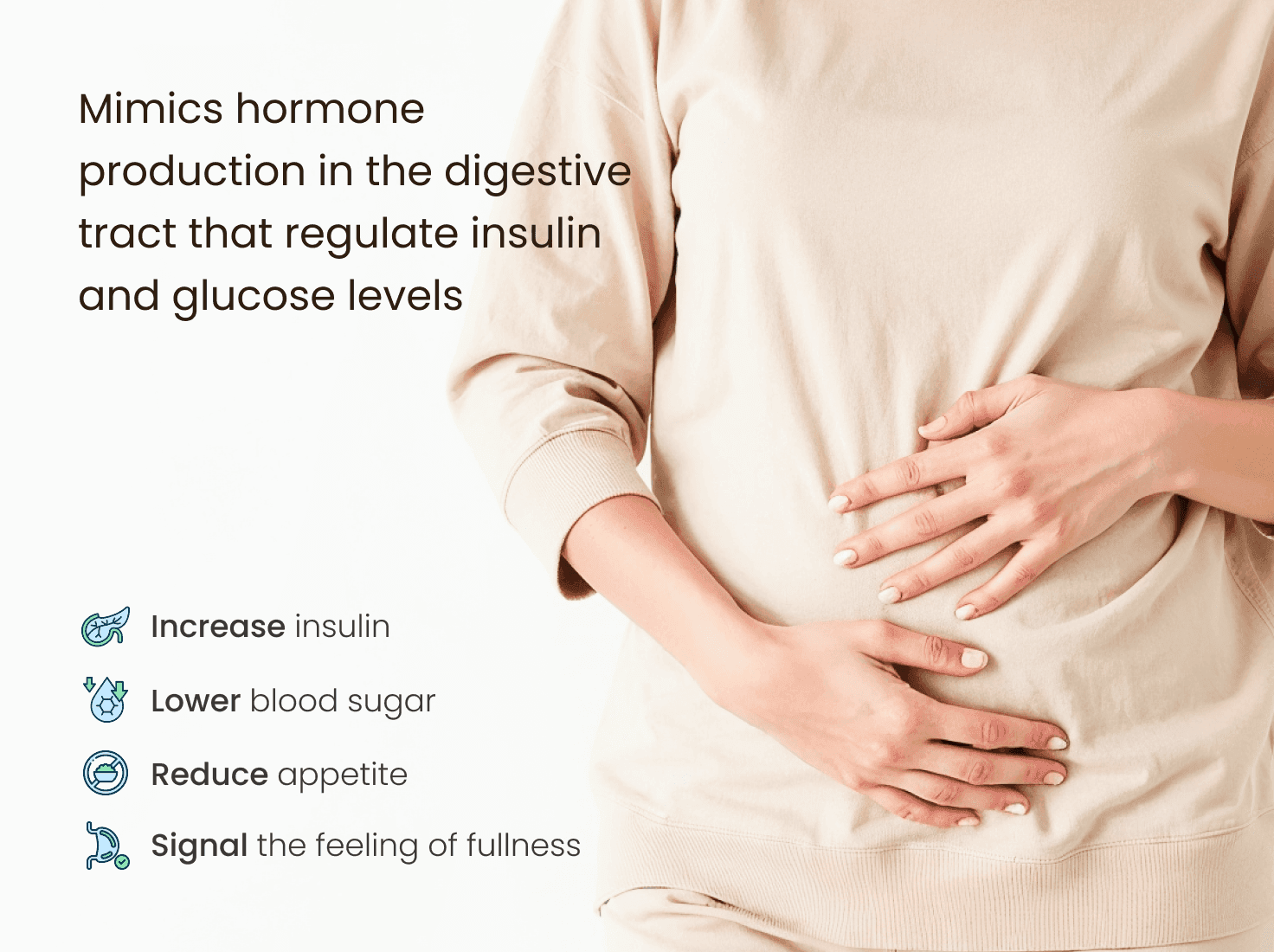
How Tirzepatide Works?
Tirzepatide is part of a group of drugs known as incretin mimetics. These drugs work in multiple ways to manage blood sugar and promote weight loss:
Increases insulin production when blood sugar is high
Slows down gastric emptying, helping you feel full for longer
Reduces glucagon, a hormone that raises blood sugar
Suppresses appetite, which can lead to less food intake
This combination of actions makes tirzepatide effective in controlling blood sugar and aiding weight loss.
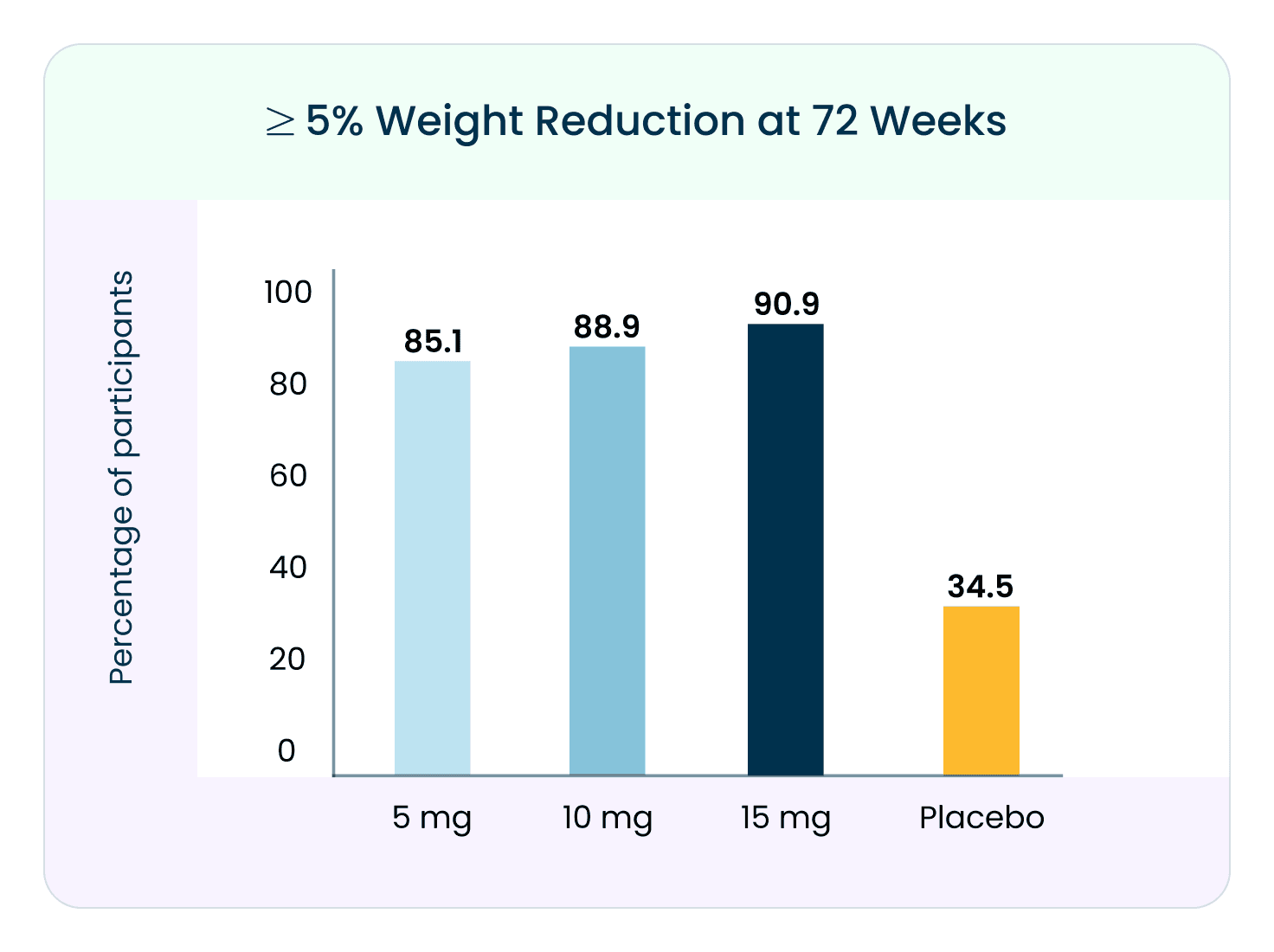
Benefits of Tirzepatide for Weight Loss³
Tirzepatide has also been extensively studied for its weight loss benefits. Two major trials involving over 2,500 participants showed promising results:
Participants took tirzepatide (5 mg, 10 mg, or 15 mg) once a week for 72 weeks.
Those who took tirzepatide experienced significant weight loss compared to those who received a placebo.
In one study, participants lost an average of 20.9% of their body weight after 72 weeks.
In August 2024, a three-year study called SURMOUNT-1 revealed that tirzepatide reduced the risk of developing type 2 diabetes by 94% in adults who were pre-diabetic and obese.
Comparison with Other Medications
Tirzepatide has been compared with other popular diabetes and weight loss medications, including:
Semaglutide
Dulaglutide
Insulin glargine
In a 2021 meta-analysis, tirzepatide outperformed these drugs in controlling blood sugar and promoting weight loss. In one trial involving nondiabetic adults with a BMI of 30 or more, those taking tirzepatide lost between 15% and 21% of their body weight, while those on a placebo lost only about 3%.
Tirzepatide Vs Semaglutide : Which is Better?⁴
The main difference between these two medications lies in their mechanism of action:
Semaglutide (found in Ozempic and Wegovy) activates only the GLP-1 receptor.
Tirzepatide (in Mounjaro) activates both GLP-1 and GIP receptors, giving it a dual-action that helps regulate metabolism more effectively.
This dual-action gives tirzepatide an edge over semaglutide in terms of both blood sugar control and weight loss.
Other Benefits of Tirzepatide⁵
Tirzepatide offers several additional health benefits beyond diabetes management and weight loss:
Dual receptor agonism (GLP-1 and GIP) enhances glucose metabolism and energy balance.
Improved glycemic control, lowering blood sugar and regulating glycogen.
Cardiovascular benefits, such as reduced blood pressure and balanced lipid levels.
Improved insulin sensitivity.
Potential improvement in Non-Alcoholic Fatty Liver Disease (NAFLD).
Better quality of life, reducing symptoms like fatigue, thirst, and frequent urination caused by uncontrolled diabetes.
Tirzepatide is also being studied for its benefits in heart failure with preserved ejection fraction (HFpEF). Early results show a 38% reduction in cardiovascular complications over two years, including reduced hospitalizations and heart failure visits.
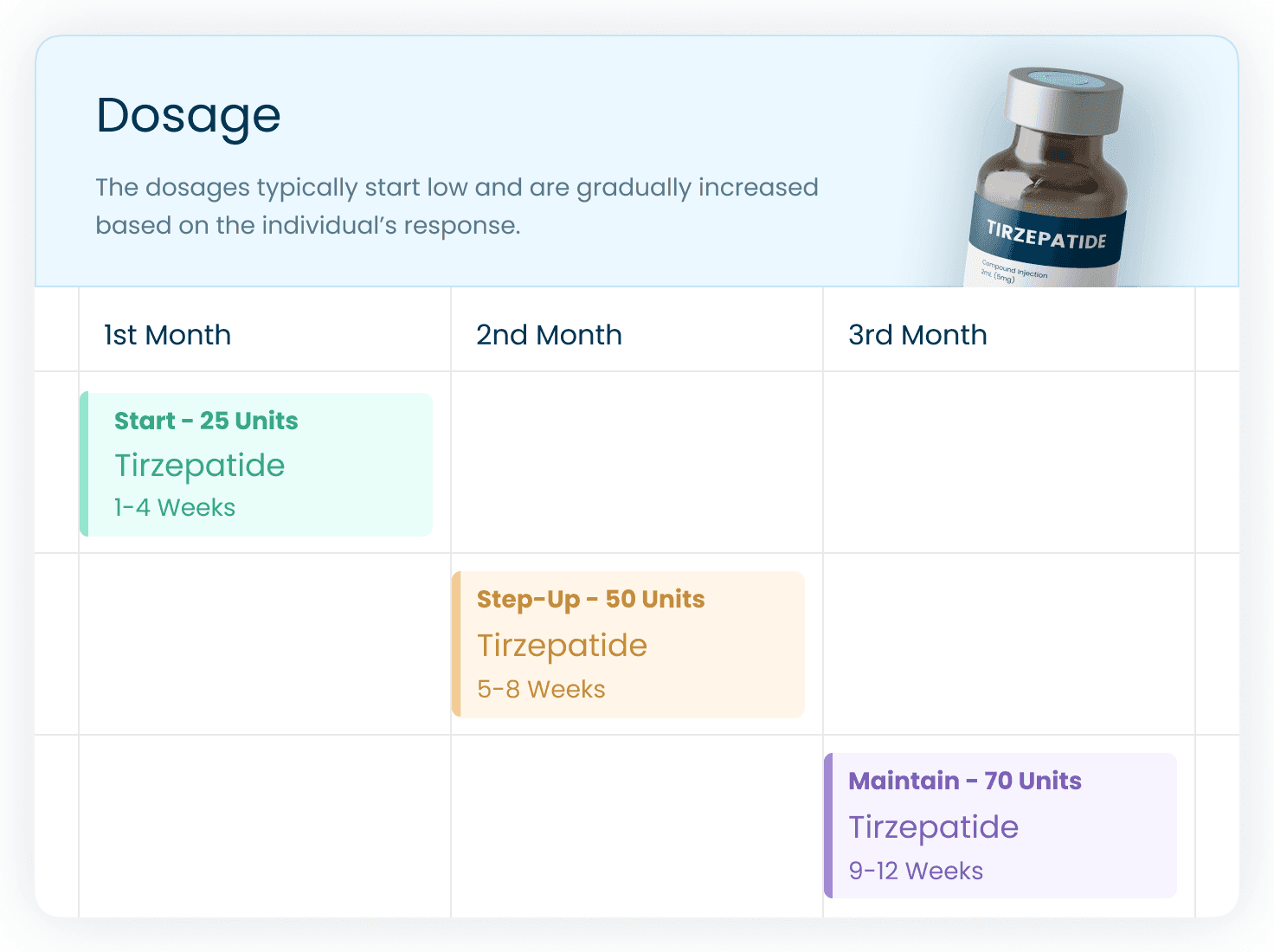
Tirzepatide Dosage and Delivery
Tirzepatide Dosage
The recommended dose of tirzepatide varies depending on the condition being treated. For type 2 diabetes and weight loss:
Start with 2.5 mg injected once a week for 4 weeks.
Your doctor may gradually increase the dose to a maximum of 15 mg per week.
Always follow your doctor’s instructions and avoid adjusting the dose without their guidance.
Tirzepatide Delivery
Tirzepatide is available in two forms:
Subcutaneous injection: Administered under the skin once a week.
Pill form: Some compounded versions may be available in pill form, though this is not FDA-approved.
Tirzepatide Weight Loss Timeline
How quickly does tirzepatide work?
Onset: It begins working within hours of the first injection.
Peak levels: Tirzepatide reaches its peak concentration in your body within 8 to 72 hours.
Half-life: The drug has a half-life of 5 days, meaning it takes about 30 days to leave your system if you stop using it.
While weight loss results vary, experts recommend aiming for a safe rate of 1 to 2 pounds per week. Studies have shown that most people start losing weight within the first few weeks of using tirzepatide.
Difference between Compounded Tirzepatide, Mounjaro and Zepbound
Mounjaro: An FDA-approved version of tirzepatide for type 2 diabetes and weight loss.
Zepbound: Another FDA-approved version of tirzepatide.
Compounded tirzepatide: Compounded drugs are prepared by state-licensed compounding pharmacies that meet FDA and state requirements, including quality standards. When compounding in compliance with federal law, compounded drugs are not subject to FDA approval and do not have to undergo safety, effectiveness, or manufacturing review.
Side effects of Tirzepatide
Most people tolerate tirzepatide well, but some side effects can occur. These include:⁶
Gastrointestinal issues: Nausea, diarrhea, and vomiting are common.
Decreased appetite: While this aids in weight loss, it can be a side effect for some.
Cardiovascular issues: Sinus tachycardia (fast heart rate) has been reported.
Renal problems: Rare cases of kidney injury due to dehydration from vomiting or diarrhea.
Dermatologic reactions: Some people experience reactions at the injection site.
Pancreatitis: GLP-1 medications, including tirzepatide, carry a small risk of pancreatitis.
Ocular complications: People with diabetic retinopathy may see worsening symptoms initially.
Serious Side Effects
Pancreatitis: Severe abdominal pain that radiates to your back.
Allergic reactions: Swelling of the face, lips, or throat, and difficulty breathing.
Kidney issues: Swelling in your legs or little to no urination.
Thyroid tumors: Lump in your neck or trouble swallowing.
If you experience any of these, contact your doctor immediately.
Who Should Use Tirzepatide?
You may be a candidate for tirzepatide if:
You have type 2 diabetes.
Your BMI is 30 or higher.
Your BMI is 27 or higher with a weight-related condition like sleep apnea, high cholesterol, or hypertension.
If you fall into one of these categories and are looking to manage your weight or diabetes, tirzepatide could be an option to consider after consulting with your healthcare provider.
Who Should Avoid Tirzepatide?
Tirzepatide is not suitable for everyone. You should avoid it if:
Patients with a history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2 (MEN-2), due to the potential risk of thyroid tumors.
Patients with known severe hypersensitivity to tirzepatide or its ingredients, especially those who have experienced allergic reactions like anaphylaxis or angioedema.
Type 1 diabetes patients: Tirzepatide is not approved for those with type 1 diabetes or other forms of diabetes, such as latent autoimmune diabetes in adults (LADA).
Tirzepatide Warnings
Animal studies have shown that tirzepatide may increase the risk of developing thyroid C-cell tumors, including medullary thyroid carcinoma.
Tirzepatide may increase the risk of thyroid C-cell tumors. If you or your family has a history of thyroid cancer, notify your doctor. Symptoms like lump or swelling in the neck, difficulty swallowing, hoarseness, or shortness of breath should be reported to your doctor immediately. Regular check-ups and lab tests may be necessary to monitor your response to the medication.
Interactions with Other Medications⁷
Tirzepatide may interact with other medications, so it's important to inform your doctor of all the medications you are taking. Here are some known interactions:
GLP-1 receptor agonists (e.g., semaglutide, liraglutide): You should not take tirzepatide along with other GLP-1 agents.
Insulin: If you are on insulin therapy, your doctor may reduce your insulin dosage to minimize the risk of hypoglycemia.
Oral contraceptives: Tirzepatide can decrease the effectiveness of oral contraceptives, so non-oral or barrier methods of contraception are recommended, especially after starting tirzepatide or during dose increases.
Oral medications: Because tirzepatide delays gastric emptying, it can affect how your body absorbs other oral medications. Caution is advised, particularly if you are on medications with a narrow therapeutic index (i.e., drugs that require precise dosing).
Conclusion
Tirzepatide is a groundbreaking medication that offers hope for people struggling with type 2 diabetes and weight management. With its dual-action mechanism targeting both GLP-1 and GIP receptors, tirzepatide offers more effective blood sugar control and weight loss compared to other medications like semaglutide. However, as with any medication, there are risks, including potential side effects and drug interactions. Always consult with your healthcare provider to determine if tirzepatide is the right option for you.
Whether you are considering tirzepatide for its diabetes management capabilities or its benefits in supporting long-term weight loss, it’s essential to follow your doctor’s instructions, be aware of potential side effects, and attend regular medical check-ups to ensure the best outcomes.
Where to get it :
Compounded Tirzepatide is available through licensed pharmacies, such as AgeMD.com. It’s important to obtain the drug from reputable sources to ensure it’s safe and effective.
Sources :
De Block, C., Bailey, C., Wysham, C., Hemmingway, A., Allen, S. E., & Peleshok, J. (2022). Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obesity and Metabolism, 25(1), 3–17. https://doi.org/10.1111/dom.14831
Pan, X., Tan, B., Chin, Y. H., Lee, E. C. Z., Kong, G., Chong, B., Kueh, M., Khoo, C. M., Mehta, A., Majety, P., Grandhi, G. R., Dimitriadis, G. K., Foo, R., Chew, N. W. S., Roux, C. W. L., Mamas, M. A., & Chan, M. Y. (2024). Efficacy and safety of tirzepatide, GLP‐1 receptor agonists, and other weight loss drugs in overweight and obesity: a network meta‐analysis. Obesity. https://doi.org/10.1002/oby.24002
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., & Stefanski, A. (2022b). Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), 205–216. https://doi.org/10.1056/nejmoa2206038
Frías, J. P., Davies, M. J., Rosenstock, J., Manghi, F. C. P., Landó, L. F., Bergman, B. K., Liu, B., Cui, X., & Brown, K. (2021). Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. New England Journal of Medicine, 385(6), 503–515. https://doi.org/10.1056/nejmoa2107519
Wadden, T. A., Chao, A. M., Machineni, S., Kushner, R., Ard, J., Srivastava, G., Halpern, B., Zhang, S., Chen, J., Bunck, M. C., Ahmad, N. N., & Forrester, T. (2023). Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nature Medicine, 29(11), 2909–2918. https://doi.org/10.1038/s41591-023-02597-w
Mishra, R., Raj, R., Elshimy, G., Zapata, I., Kannan, L., Majety, P., Edem, D., & Correa, R. (2023). Adverse events related to tirzepatide. Journal of the Endocrine Society, 7(4). https://doi.org/10.1210/jendso/bvad016
Current version
Oct 7, 2024
Written by
Eileen Quinones (Certified Family Nurse Practitioner)
Fact checked by
Dr. Joel Lopez (MD, CNS, DABAARM)
Lose weight effectively
with GLP-1s
Fill out a quick form to share your
medical history, helping us tailor the
perfect plan for you.

Tirzepatide 101: Uses, Benefits, Side Effects, and Comparison to Semaglutide
Tirzepatide

Eileen Quinones
•
10 mins read
• Oct 7, 2024
Tirzepatide is gaining attention for its effectiveness in managing type 2 diabetes and supporting weight loss. This blog will walk you through what tirzepatide is, how it works, its benefits, side effects, and how it compares to other medications like semaglutide. Whether you're considering this medication for diabetes management or weight loss, here's everything you need to know about the peptide.
What is Tirzepatide?
Tirzepatide is a synthetic antidiabetic drug used to treat type 2 diabetes. It belongs to a new class of drugs called dual GLP-1 and GIP receptor agonists. This means it activates two types of hormone receptors:
GLP-1 (glucagon-like peptide-1)
GIP (glucose-dependent insulinotropic peptide)
These hormones, called incretins, help your body release insulin when blood sugar is high. Because it acts on two receptors, tirzepatide is often referred to as a "twincretin."
Tirzepatide was approved by the FDA in 2022 under the brand name Mounjaro. It is administered via an injection under the skin once a week.
History of Tirzepatide
Tirzepatide was developed by Eli Lilly for managing type 2 diabetes and weight loss. The drug was first patented in 2016, and after successful trials, Eli Lilly applied for FDA approval in October 2021. By April 2022, tirzepatide was shown to help not just diabetes patients but also overweight and obese individuals—regardless of whether they had diabetes.
Clinical Trials and FDA Approval
Tirzepatide received FDA approval based on data from nine clinical trials involving over 7,700 participants with type 2 diabetes. These trials were conducted worldwide, including in the U.S., Brazil, Japan, and the EU.
Five trials focused on how well tirzepatide controlled blood sugar.¹
Four trials focused on its safety.²
Participants were given tirzepatide, a placebo, or other diabetes medications. These trials lasted between 40 to 104 weeks, measuring the reduction in HbA1c, which tracks long-term blood sugar control.
How Tirzepatide Works?
Tirzepatide is part of a group of drugs known as incretin mimetics. These drugs work in multiple ways to manage blood sugar and promote weight loss:
Increases insulin production when blood sugar is high
Slows down gastric emptying, helping you feel full for longer
Reduces glucagon, a hormone that raises blood sugar
Suppresses appetite, which can lead to less food intake
This combination of actions makes tirzepatide effective in controlling blood sugar and aiding weight loss.
Benefits of Tirzepatide for Weight Loss³
Tirzepatide has also been extensively studied for its weight loss benefits. Two major trials involving over 2,500 participants showed promising results:
Participants took tirzepatide (5 mg, 10 mg, or 15 mg) once a week for 72 weeks.
Those who took tirzepatide experienced significant weight loss compared to those who received a placebo.
In one study, participants lost an average of 20.9% of their body weight after 72 weeks.
In August 2024, a three-year study called SURMOUNT-1 revealed that tirzepatide reduced the risk of developing type 2 diabetes by 94% in adults who were pre-diabetic and obese.
Comparison with Other Medications
Tirzepatide has been compared with other popular diabetes and weight loss medications, including:
Semaglutide
Dulaglutide
Insulin glargine
In a 2021 meta-analysis, tirzepatide outperformed these drugs in controlling blood sugar and promoting weight loss. In one trial involving nondiabetic adults with a BMI of 30 or more, those taking tirzepatide lost between 15% and 21% of their body weight, while those on a placebo lost only about 3%.
Tirzepatide Vs Semaglutide : Which is Better?⁴
The main difference between these two medications lies in their mechanism of action:
Semaglutide (found in Ozempic and Wegovy) activates only the GLP-1 receptor.
Tirzepatide (in Mounjaro) activates both GLP-1 and GIP receptors, giving it a dual-action that helps regulate metabolism more effectively.
This dual-action gives tirzepatide an edge over semaglutide in terms of both blood sugar control and weight loss.
Other Benefits of Tirzepatide⁵
Tirzepatide offers several additional health benefits beyond diabetes management and weight loss:
Dual receptor agonism (GLP-1 and GIP) enhances glucose metabolism and energy balance.
Improved glycemic control, lowering blood sugar and regulating glycogen.
Cardiovascular benefits, such as reduced blood pressure and balanced lipid levels.
Improved insulin sensitivity.
Potential improvement in Non-Alcoholic Fatty Liver Disease (NAFLD).
Better quality of life, reducing symptoms like fatigue, thirst, and frequent urination caused by uncontrolled diabetes.
Tirzepatide is also being studied for its benefits in heart failure with preserved ejection fraction (HFpEF). Early results show a 38% reduction in cardiovascular complications over two years, including reduced hospitalizations and heart failure visits.
Tirzepatide Dosage and Delivery
Tirzepatide Dosage
The recommended dose of tirzepatide varies depending on the condition being treated. For type 2 diabetes and weight loss:
Start with 2.5 mg injected once a week for 4 weeks.
Your doctor may gradually increase the dose to a maximum of 15 mg per week.
Always follow your doctor’s instructions and avoid adjusting the dose without their guidance.
Tirzepatide Delivery
Tirzepatide is available in two forms:
Subcutaneous injection: Administered under the skin once a week.
Pill form: Some compounded versions may be available in pill form, though this is not FDA-approved.
Tirzepatide Weight Loss Timeline
How quickly does tirzepatide work?
Onset: It begins working within hours of the first injection.
Peak levels: Tirzepatide reaches its peak concentration in your body within 8 to 72 hours.
Half-life: The drug has a half-life of 5 days, meaning it takes about 30 days to leave your system if you stop using it.
While weight loss results vary, experts recommend aiming for a safe rate of 1 to 2 pounds per week. Studies have shown that most people start losing weight within the first few weeks of using tirzepatide.
Difference between Compounded Tirzepatide, Mounjaro and Zepbound
Mounjaro: An FDA-approved version of tirzepatide for type 2 diabetes and weight loss.
Zepbound: Another FDA-approved version of tirzepatide.
Compounded tirzepatide: Compounded drugs are prepared by state-licensed compounding pharmacies that meet FDA and state requirements, including quality standards. When compounding in compliance with federal law, compounded drugs are not subject to FDA approval and do not have to undergo safety, effectiveness, or manufacturing review.
Side effects of Tirzepatide
Most people tolerate tirzepatide well, but some side effects can occur. These include:⁶
Gastrointestinal issues: Nausea, diarrhea, and vomiting are common.
Decreased appetite: While this aids in weight loss, it can be a side effect for some.
Cardiovascular issues: Sinus tachycardia (fast heart rate) has been reported.
Renal problems: Rare cases of kidney injury due to dehydration from vomiting or diarrhea.
Dermatologic reactions: Some people experience reactions at the injection site.
Pancreatitis: GLP-1 medications, including tirzepatide, carry a small risk of pancreatitis.
Ocular complications: People with diabetic retinopathy may see worsening symptoms initially.
Serious Side Effects
Pancreatitis: Severe abdominal pain that radiates to your back.
Allergic reactions: Swelling of the face, lips, or throat, and difficulty breathing.
Kidney issues: Swelling in your legs or little to no urination.
Thyroid tumors: Lump in your neck or trouble swallowing.
If you experience any of these, contact your doctor immediately.
Who Should Use Tirzepatide?
You may be a candidate for tirzepatide if:
You have type 2 diabetes.
Your BMI is 30 or higher.
Your BMI is 27 or higher with a weight-related condition like sleep apnea, high cholesterol, or hypertension.
If you fall into one of these categories and are looking to manage your weight or diabetes, tirzepatide could be an option to consider after consulting with your healthcare provider.
Who Should Avoid Tirzepatide?
Tirzepatide is not suitable for everyone. You should avoid it if:
Patients with a history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2 (MEN-2), due to the potential risk of thyroid tumors.
Patients with known severe hypersensitivity to tirzepatide or its ingredients, especially those who have experienced allergic reactions like anaphylaxis or angioedema.
Type 1 diabetes patients: Tirzepatide is not approved for those with type 1 diabetes or other forms of diabetes, such as latent autoimmune diabetes in adults (LADA).
Tirzepatide Warnings
Animal studies have shown that tirzepatide may increase the risk of developing thyroid C-cell tumors, including medullary thyroid carcinoma.
Tirzepatide may increase the risk of thyroid C-cell tumors. If you or your family has a history of thyroid cancer, notify your doctor. Symptoms like lump or swelling in the neck, difficulty swallowing, hoarseness, or shortness of breath should be reported to your doctor immediately. Regular check-ups and lab tests may be necessary to monitor your response to the medication.
Interactions with Other Medications⁷
Tirzepatide may interact with other medications, so it's important to inform your doctor of all the medications you are taking. Here are some known interactions:
GLP-1 receptor agonists (e.g., semaglutide, liraglutide): You should not take tirzepatide along with other GLP-1 agents.
Insulin: If you are on insulin therapy, your doctor may reduce your insulin dosage to minimize the risk of hypoglycemia.
Oral contraceptives: Tirzepatide can decrease the effectiveness of oral contraceptives, so non-oral or barrier methods of contraception are recommended, especially after starting tirzepatide or during dose increases.
Oral medications: Because tirzepatide delays gastric emptying, it can affect how your body absorbs other oral medications. Caution is advised, particularly if you are on medications with a narrow therapeutic index (i.e., drugs that require precise dosing).
Conclusion
Tirzepatide is a groundbreaking medication that offers hope for people struggling with type 2 diabetes and weight management. With its dual-action mechanism targeting both GLP-1 and GIP receptors, tirzepatide offers more effective blood sugar control and weight loss compared to other medications like semaglutide. However, as with any medication, there are risks, including potential side effects and drug interactions. Always consult with your healthcare provider to determine if tirzepatide is the right option for you.
Whether you are considering tirzepatide for its diabetes management capabilities or its benefits in supporting long-term weight loss, it’s essential to follow your doctor’s instructions, be aware of potential side effects, and attend regular medical check-ups to ensure the best outcomes.
Where to get it :
Compounded Tirzepatide is available through licensed pharmacies, such as AgeMD.com. It’s important to obtain the drug from reputable sources to ensure it’s safe and effective.




Current version
Oct 7, 2024
Written by
Eileen Quinones (Certified Family Nurse Practitioner)
Fact checked by
Dr. Joel Lopez (MD, CNS, DABAARM)
Lose weight effectively
with GLP-1s
Fill out a quick form to share your
medical history, helping us tailor the
perfect plan for you.

Lose weight effectively
with GLP-1s
Fill out a quick form to share your
medical history, helping us tailor the
perfect plan for you.

Sources :
De Block, C., Bailey, C., Wysham, C., Hemmingway, A., Allen, S. E., & Peleshok, J. (2022). Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obesity and Metabolism, 25(1), 3–17. https://doi.org/10.1111/dom.14831
Pan, X., Tan, B., Chin, Y. H., Lee, E. C. Z., Kong, G., Chong, B., Kueh, M., Khoo, C. M., Mehta, A., Majety, P., Grandhi, G. R., Dimitriadis, G. K., Foo, R., Chew, N. W. S., Roux, C. W. L., Mamas, M. A., & Chan, M. Y. (2024). Efficacy and safety of tirzepatide, GLP‐1 receptor agonists, and other weight loss drugs in overweight and obesity: a network meta‐analysis. Obesity. https://doi.org/10.1002/oby.24002
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., & Stefanski, A. (2022b). Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), 205–216. https://doi.org/10.1056/nejmoa2206038
Frías, J. P., Davies, M. J., Rosenstock, J., Manghi, F. C. P., Landó, L. F., Bergman, B. K., Liu, B., Cui, X., & Brown, K. (2021). Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. New England Journal of Medicine, 385(6), 503–515. https://doi.org/10.1056/nejmoa2107519
Wadden, T. A., Chao, A. M., Machineni, S., Kushner, R., Ard, J., Srivastava, G., Halpern, B., Zhang, S., Chen, J., Bunck, M. C., Ahmad, N. N., & Forrester, T. (2023). Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nature Medicine, 29(11), 2909–2918. https://doi.org/10.1038/s41591-023-02597-w
Mishra, R., Raj, R., Elshimy, G., Zapata, I., Kannan, L., Majety, P., Edem, D., & Correa, R. (2023). Adverse events related to tirzepatide. Journal of the Endocrine Society, 7(4). https://doi.org/10.1210/jendso/bvad016